Lensless Microscopy
Gaboscope
Optical microscopy is essential in biomedical and clinical research facilitating “seeing is believing” paradigm, capacitating diagnostically crucial examination of cells/tissues. It is under constant development with a goal of enhanced resolution, contrast, depth, speed, and information content, with computational techniques emerging recently as capable solutions (e.g., deep learning). Fluorescence microscopy is the most impactful microscopic technique. It relies on labeling the structure of interest with fluorophores – nanoscopic light-bulbs attached to the specimen rendering its high-contrast images. Despite their gigantic success, all fluorescence methods have inherent limitations: (1) time-consuming fluorescence labeling is prone to phototoxicity and (2) it alters normal physiological processes invasively affecting natural cell-behavior jeopardizing the reliability of the study. Thus, a strong demand on capable label-free methods arises with a goal of studying the dynamics of live cells and their natural physiological activities. The sample is not chemically stained and therefore can be imaged basing on its endogenous contrast agents, i.e., internal absorption, scattering or refractive index (phase delay). Zernike/Nomarski phase contrast microscopy are well-known marker-free methods, but they suffer from halo and low contrast and do not provide quantitative phase information, thus are not suitable for standardized analysis. Quantitative Phase Microscopy (QPM) stands out among modern label-free imaging techniques as extremely capable high-contrast approach based on the interference measurement of sample refractive index distribution. Although application-oriented research provides exciting advancements, QPM benchtop devices tend to be bulky and costly, both in financial terms and in effort required to master them. They are based on a modified brightfield microscope, thus possess limitations in terms of finite microscope objective numerical aperture and small depth of focus.
These disadvantages are bypassed by the lensfree holographic microscopy (LHM), which in easy-to-assemble manner (light source+specimen+camera) captures a “holographic shadow” of the illuminated object. This “shadow” is a Gabor in-line hologram generated upon interference of non-scattered (reference) and object scattered wave-fronts. Numerical reconstruction opens up possibilities to study extremely large volumes (thousands of cubic millimeters) without the depth of focus limitations, which is a fantastic advantage of LHM. The problem to be solved within the GaboScope is connected with the fact that classical schemes employed to recover, upon backpropagation, the in-focus objects are very sensitive to low-quality of holograms (strong noise and incoherent background, low contrast, high sample density and scattering etc.) and apply time-consuming algorithmic/experimental solutions which impede deterministic high-throughput marker-free investigation of dynamic processes in a large holographically rendered volume.
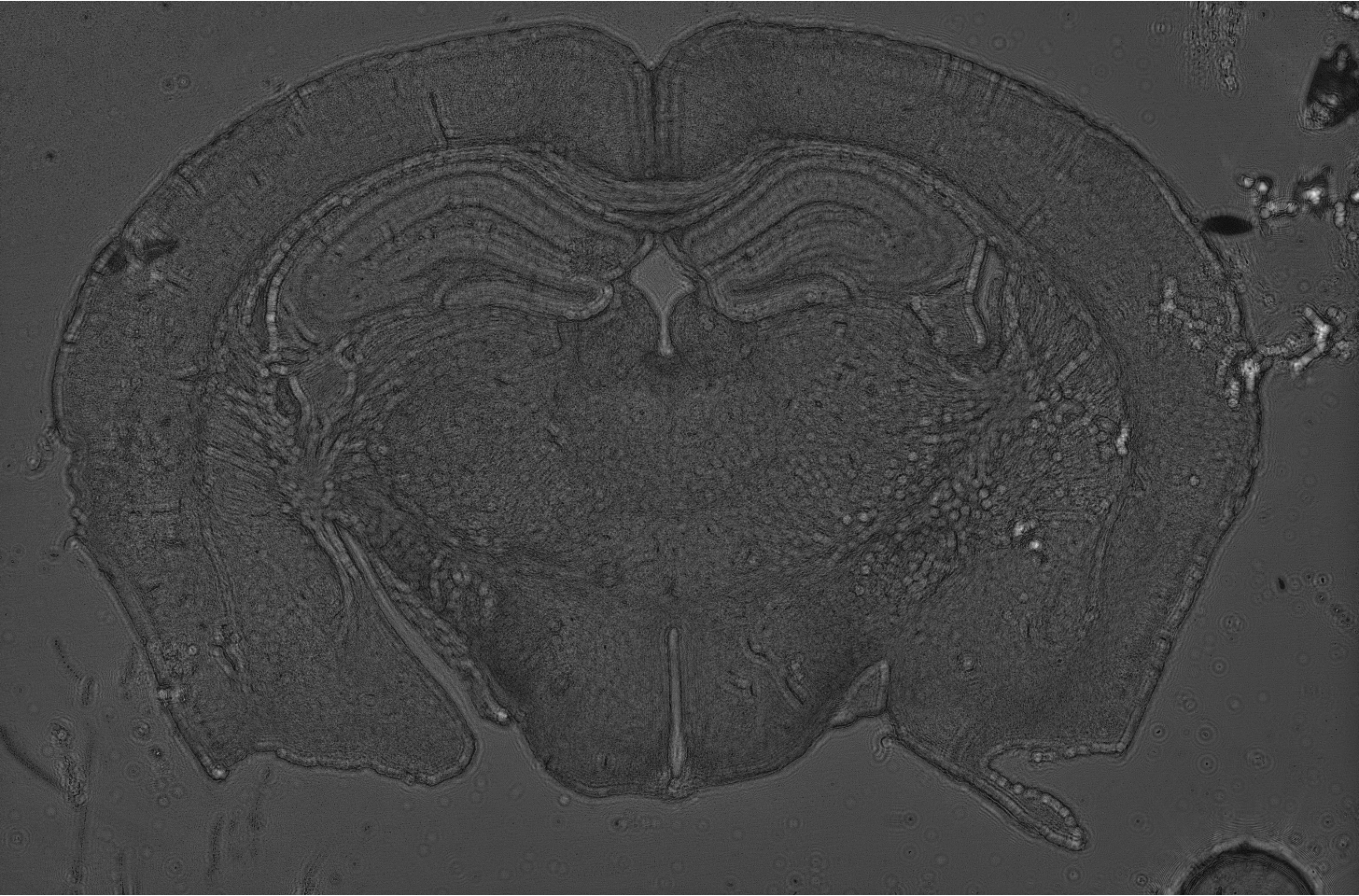
Mouse brain slice reconstruted from Gabor hologram.
Hologram was registered in lensless holographic microscopy setup.
In the GaboScope we unprecedentedly aim at advancing the LHM in terms of high-throughput label-free large-volume bio-imaging of dynamic live cells with algorithmic specificity in hologram low-SNR regimes. Thus, a new class of robust fast non-iterative 4D phase/amplitude reconstruction methods will be established and released publicly to set a stage for democratizing high-end lensfree microscopy (preferably on low-cost 3D printed custom setups). The GaboScope has unique features in terms of international network of excellence-driven partner institutions and very detailed scientific novelty-driven plan. Three pioneering aspects are envisioned.
- Pushing the numerical holographic reconstruction limits in terms augmented dark-field and phase signal-to-noise ratio in low-light-dosage conditions and accounting for twin-image removal, spherical propagation and multiple-scattering.
- Developing deep learning based solutions for fast and accurate numerical labelling, counting and 4D tracking of the diversified dynamic objects in large holographically rendered volumes; democratizing them through open-access releases with a possibility to user-driven transfer learning and fine-tuning the network to specific needs.
- Enabling unique applications in challenging biomedical imaging through novel increased time-space-bandwidth product setups and lens-free tomographic reconstruction for disambiguating the refractive index structure and biological thickness.